Move Over Truvada! FDA Approves Apretude, First Injectable HIV Prevention Drug
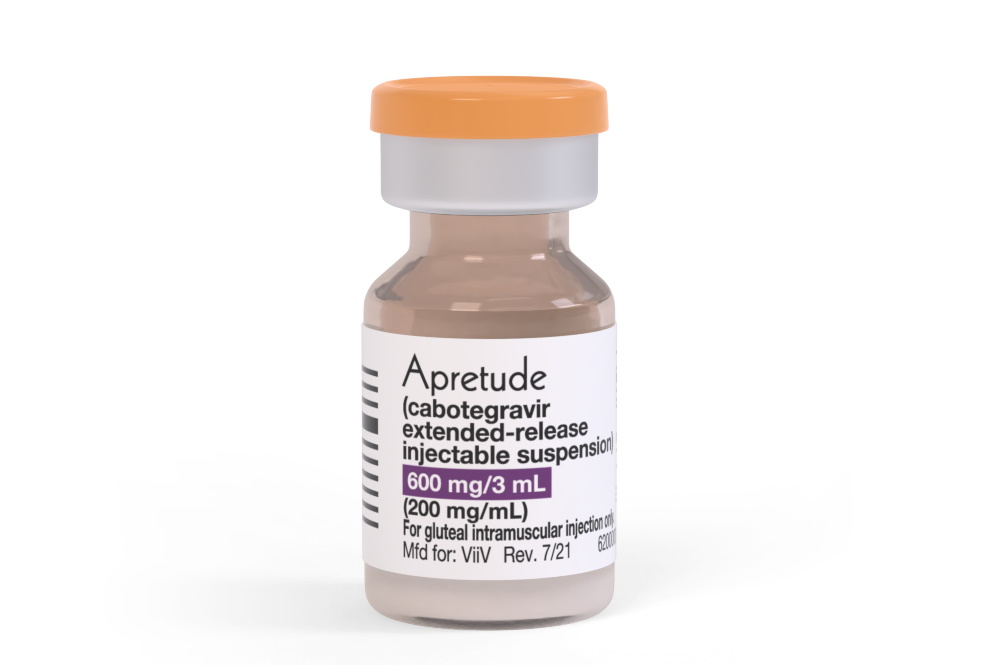 If you’re tired of taking pills every day, now you can receive an injectable drug to prevent HIV:
If you’re tired of taking pills every day, now you can receive an injectable drug to prevent HIV:
The Food and Drug Administration has approved the first long-acting injectable medication for use as pre-exposure prevention, or PrEP, against HIV, the agency announced Monday.
Apretude, the new drug, is an injectable given every two months as an alternative to HIV prevention pills, like Truvada and Descovy, which have been shown to reduce the risk of HIV by 99 percent when taken daily.
Two FDA trials analyzing the safety and efficacy of the novel drug found that Apretude was more likely to reduce HIV than the daily oral medications — by 69 percent for cisgender men and transgender women who have sex with men and by 90 percent for cisgender women. Apretude’s superior efficacy was apparently driven by the greater ease with which study participants adhered to the every-other-month regimen compared with taking a pill every day.
You’ve already been pricked with three (or four, or maybe five by next year) injections of a COVID vaccine, so being jabbed six more times per year with another new drug won’t kill you.
[NBC: FDA approves first injectable HIV prevention drug]